Reagent analysis

No imine structure yet

No nucleophile structure yet
BINOP Catalyst Optimizer
Jolene P. Reid, Kristaps Ermanis and Jonathan M. Goodman
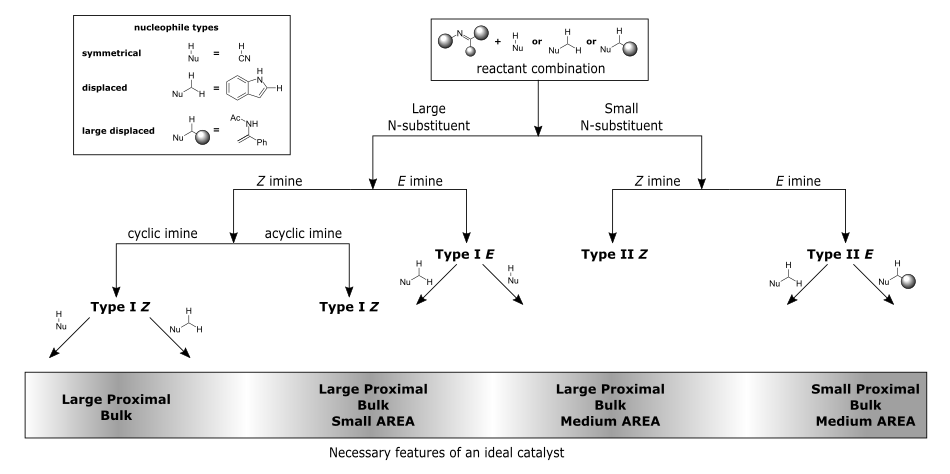
This is a web application implementing the recently developed algorithm for the selection of optimal BINOL-derived phosphoric acid catalysts for enantioselective additions to imine. The rules were developed from extensive computational DFT studies and parametrisation. This applet uses JavaScript Molecular Editor (JSME) for structure input and RDKit for structure analysis. The full code of this applet is available on GitHub.
Structure input
Instructions
Input the structure of either imine or the nucleophile using the structure editor to the left and press either "Input imine structure" or "Input nucleophile structure", as appropriate. When both structures have been input, the results from the reagent, reaction and catalyst analysis will be shown below.
The model is quite general and covers most of enantioselective imine addition reactions found in literature and also gives educated guesses about many that haven't yet been reported. Having said that, the applet will not provide an answer in situations where answer cannot be given with any certainty.
N-substituents recognized: Ac, Bn, Bz, aryl, PMP, Boc, Cbz, Ts and general cyclic imines.
Nucleophile classes recognized: HCN, alcohols, peroxides, primary amines, thiols, Hantzsch esters, phtalimides, diazoesters, diazoamides, diazophosphonates, phosphonates, benzthiazolines, pyrrole containing heterocyclic compounds, various enols and enamides.
As JSME will always convert neighbouring opposite charges to a bond, please use a triple bond to input diazo compounds.
Results below
Reagent analysis

No imine structure yet

No nucleophile structure yet
Reaction and catalyst analysis
No reactants input yet
Suggested catalysts

Decision tree